
Open frame SMPS Medical Power Supply
- Transportation:
- Ocean, Air
- Port:
- ShenZhen
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | ShenZhen |
|---|---|
| Payment Type: | T/T,Paypal |
| Incoterm: | FOB,EXW |
| Certificate: | ISO9001:2015 ,EN ISO 13485:2016 |
| Transportation: | Ocean,Air |
| Port: | ShenZhen |
Longxc Smelted the industry's first professional dental medical power supply, first medical adapter with LED, first slim ultra small 90W adapter and so on. Gradually break through technical barriers to meet the power requirements of all kinds of medical equipment. High quality products derive from strict control of production processes.
And Provide unique systems security EMC solutions and training services. From components to end items, from pre-sales to after-sales for each product, Longxc Medical power supply pursue every detail: precision casting product, character makes product, quality accomplish product, to achieve products of quality, to serve customers with technology, Serving customers with technology. Ensure that equipment are safer, more stable, and more efficient.
Feature
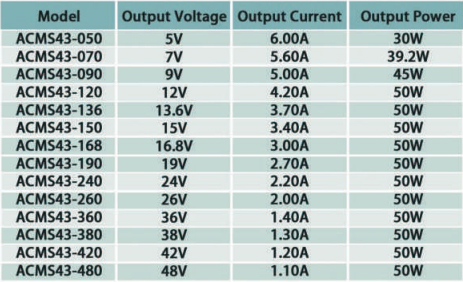
Model:ACMS43
Class II Construction
Output Voltage:5-48Vdc
Output Peak Power:85W
OCP/OVP/SCP
Size(mm):76.2(L)*50.8(W)*21(H)
Terminal Output
Delivery Guarantee:The company has a team of over 200 people and a modern dust-free workshop of 5000 square meters. It uses six sets of fully intelligent system equipment and automated production lines both domestically and internationally, and has passed ISO14000/ISO9001/ISO13485 management system certification. It has also obtained multiple qualification certifications such as TUV/UL/CE/CB, ensuring timely delivery.
With the release of the Medical Device Supervision and Administration Regulations (State Council Order No. 739) in 2021, the comprehensive update of medical device regulations and the introduction of industry regulations are about to begin; As a production enterprise and research and development institution, strictly implementing the "Medical Device Production Quality Management Standards" and its on-site inspection principles in accordance with regulations is the most basic requirement. It is a basic guarantee for enterprise practitioners to learn, master and apply standardized professional knowledge and skills. To promote the development of the medical device industry and help various medical device enterprises and research and development institutions comprehensively improve their quality management and guarantee capabilities, the association plans to hold training for medical device GMP specialists and internal auditors.
1. The person in charge and representative of management of medical device enterprises;
2. Technical personnel from medical device research and development institutions;
3. Personnel related to the quality management system of medical device manufacturing enterprises;
4. Personnel intended to work in the quality management system of medical devices;
5. Other students or individuals interested in the field of medical devices;
Related Keywords









